Abhay Satoskar
Professor of Pathology and Microbiology
257 Evans Hall
Areas of Expertise
- Immune mechanisms
Education
- Ph.D. University of Strathclyde-Glasgow
- MD, University of Bombay
Abhay Satoskar, MD, PhD is a Professor in the Division of Experimental Pathology and Department of Microbiology. Dr. Satoskar’s interests are immunology and infectious disease with a focus on parasite immunology.
Research
Over 12 million people currently suffer from leishmaniasis, and approximately 2 million new cases occur annually, making it a major global health problem and a WHO classified neglected tropical disease (NTD). Cutaneous leishmaniasis (CL) is the most common form of Leishmania infection, which is now endemic in the United States, manifests as localized skin lesions that can become chronic, leading to significant tissue destruction and disfigurement.

Other forms of infections are mucosal leishmaniasis (ML), or life-threatening visceral leishmaniasis (VL) caused by L. donovani and L. infantum which is characterized by dissemination of the parasites to the liver, spleen and bone marrow. Leishmania are obligate intracellular parasites that invade and multiply within host phagocytes, which are also the effector cells involved in parasite elimination However, the mechanisms of parasite survival and persistence in the phagocytes in are not clear. The research in our laboratory is focused on understanding the mechanisms of immune evasion and parasite persistence in different forms of leishmaniasis using experimental models and in humans and develop novel therapies and a vaccine for global elimination of this disease. We are also interested in elucidating the mechanisms of sex-determined susceptibility and bystander immune suppression in leishmaniasis using experimental models.
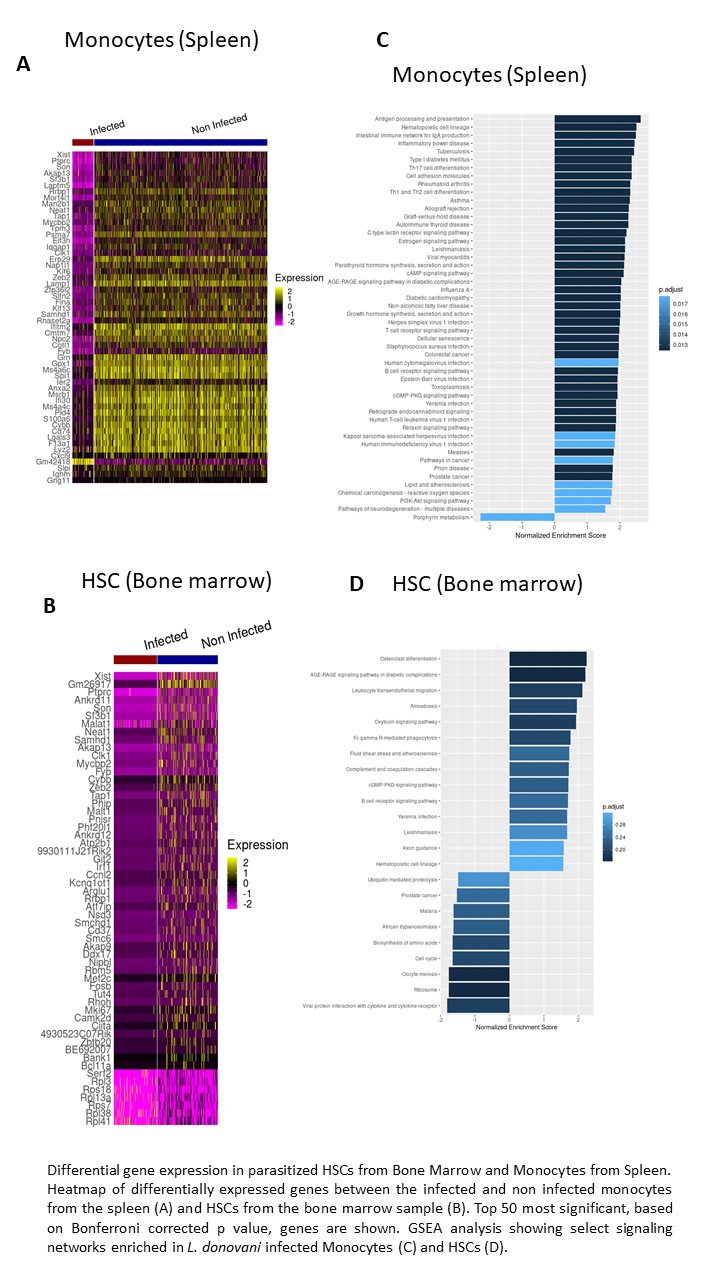
Mechanisms of immune evasion and parasite persistence: Using an experimental animal model of VL an approaches such imaging flow cytometry, we have discovered that Ly6Chi inflammatory monocytes (iMOs) recruited to the organs after L. donovani infection are preferred targets for the parasites and they play a role in disease pathogenesis (Scientific Reports-Nature. 2017; 7 1:14693). We found that blockade of iMO influx into organs reduces generation pathogenic IL-10-producing CD4+Th1 cells in the spleen and accelerates parasite clearance. Although phagocytes were thought to be the exclusive reservoir of parasites, by performing a dual single cell RNAseq (scRNASeq) of cells from the organs of chronically infected mice we have discovered several rare and uncommon cell types that could be infected by L. donovani (Cell Rep. 2023 Sep 26;42(9):113097). In particular, we found that Hematopoietic Stem Cells (HSCs), constitute the predominant reservoir of parasites in the bone marrow during chronic VL whereas monocytes are the reservoirs in the spleen as reported previously. Analysis of scRNAseq data have discovered several host genes and pathways that upregulated in the infected HSCs and could be involved in promoting parasite persistence in these cells. Studies are underway to evaluate the roles of these pathways in pathogenesis of VL. The discovery of HSCs and other cell types as potential reservoirs of parasites in organs of experimentally infected mice represents an important advance to investigate the role of these cells in parasite persistence and disease pathogenesis. We are now using similar approaches to identify and characterize the host cells that are reservoirs of parasites in the bone marrow and spleen of VL patients from disease endemic countries, and to discover host pathways that may promote parasite persistence.
Mechanisms of sex-associated resistance to leishmaniasis: Sex-associated differences in resistance to bacterial, viral, and parasitic infections have been described in epidemiological and experimental studies. Females are more prone to develop autoimmune and inflammatory diseases which is which is generally associated with increased T cell activation and higher production of Th1 cytokines such as IFN-g, but they are also more resistant to infections. Although gender-biased differences have been attributed to effects of sex hormones on the immune system, immune system genes located on X chromosome also appear to play a role. Females have two X chromosomes (XX) and hence to equalize gene expression between sexes, they undergo a process called X chromosome inactivation (XCI) transcriptionally silence randomly chosen one X chromosome. However, it is known that a number of X-linked genes escape XCI and display biallelic gene expression, and thus contributing to increased inflammatory response in females. One such gene encodes chemokine receptor CXCR3, which is expressed many cells such as NK cells, Th1 cells and is critical for their recruitment to tackle infection. By developing a novel CXCR3 dual reporter mouse to visualize CXCR3 allelic expression, we found that CXCR3 escapes XCI, biallelic CXCR3-expressing T cells display hyper-inflammatory phenotype compared with T cells that express CXCR3 from one allele during Leishmania mexicana infection (J Immunol (2019) 203 (4): 789–794). These reporter mice also lead to discovery of two distinct populations of innate CD8 T cells (J Immunol (2013) 190 (5): 2229–2240; FASEB J. 2015 Mar;29(3):1019-28). Ongoing studies in the lab are undertaking further characterization of biallelic CXCR3-expressing T cells and innate CD8+ T cells and investigate their roles in immune homeostasis.
Development of novel treatments and a vaccine for leishmaniasis: Treatments that are currently available for leishmaniasis have significant issues such as prolonged administration by injection, emergence of drug resistance and toxicity that leads to poor patient compliance. Therefore, there is need for safer and effective drugs for leishmaniasis. Furthermore, currently there is no vaccine available against leishmaniasis in humans. A vaccine is indispensable for elimination of leishmaniasis. Our research in collaboration with college of Pharmacy has led to discovery of a novel anti-leishmanial compound pentalinonsterol (PEN) from a natural product used by traditional Myana healers to treat leishmaniasis (ACS Infect. Dis. 2015, 1, 10, 497–506; J Nat Prod. 2017 Sep 6;80(9):2515–2523; Cell Biochem Biophys. 2022 Mar;80(1):45-61). We have found that PEN also has immunomodulatory properties and hence could be used in other diseases. Studies are underway to determine mechanism of anti-parasitic and immunomodulatory actions of PEN. Other studies from our lab are focused on investing whether host factors and pathways that promote disease susceptibility could be targets for Host directed therapies (HDTs) for leishmaniasis. These studies have shown that anti-cancer drugs targeting host factors such as PI3Kɣ and Btk/Itk can be repurposed for treating leishmaniasis (Blood. 2008 Jul 24;112(8):3048–305; Proc Natl Acad Sci U S A. 2012 Jan 9;109(4):1251–1256; Blood. 2013 Oct 10;122(15):2539-49; The Journal of Infectious Diseases, Volume 219, Issue 4, 15 February 2019, Pages 599–608). Other translational projects involve evaluating safety and efficacy of topical field -deployable and affordable therapies for treating CL in the developing countries (Br J Dermatol. 2013 May;168(5):1114-9; The Lancet Volume 377, Issue 9765, p610February 12, 2011) A consortium of international scientists led by our group has also developed a live attenuated vaccine for leishmaniasis using CRISPR technology (Nature Communications volume 11, Article number: 3461 (2020); Commun Biol. 2021 Jul 30;4(1):929; NPJ Vaccines. 2022 Mar 2;7(1):32; NPJ Vaccines. 2022 Dec 3;7(1):157; NPJ Vaccines volume 9, Article number: 250 (2024)). This vaccine has demonstrated excellent safety and efficacy against CL and VL in pre-clinical testing and is currently under clinical development for human trials. Current studies are focused on determining vaccine induced mechanisms of protection and discovering biomarkers of vaccine-induced immunity using animal models and clinical samples.