
Krithika Rajaram
Assistant Professor of Microbiology
440A Biological Sciences Building
Research Lab:
Rooms 440, 444, and 446
Areas of Expertise
- Parasitology
- Microbial Physiology
Education
- Postdoctoral Fellowship, Johns Hopkins University, 2020
- Ph.D., Indiana University Bloomington, 2015
- B.Tech., Anna University, India; 2008
Research Interests
Malaria parasite metabolism: Malaria is an ancient illness that continues to impact millions of people every year. The most dangerous form of this disease is caused by a unicellular parasite called Plasmodium falciparum that spreads from one human to another via a mosquito vector. Infected individuals begin to display malaria symptoms after the parasites invade and multiply within their red blood cells (RBCs). Our lab is interested in determining how P. falciparum sustains itself during this clinically relevant phase of disease – does the parasite acquire essential metabolites from the host RBC, or does it rely heavily on its own biosynthetic pathways? Using a combination of genetic, biochemical and metabolomic techniques, we hope to pinpoint metabolic vulnerabilities in P. falciparum that could serve as targets for therapeutic intervention.
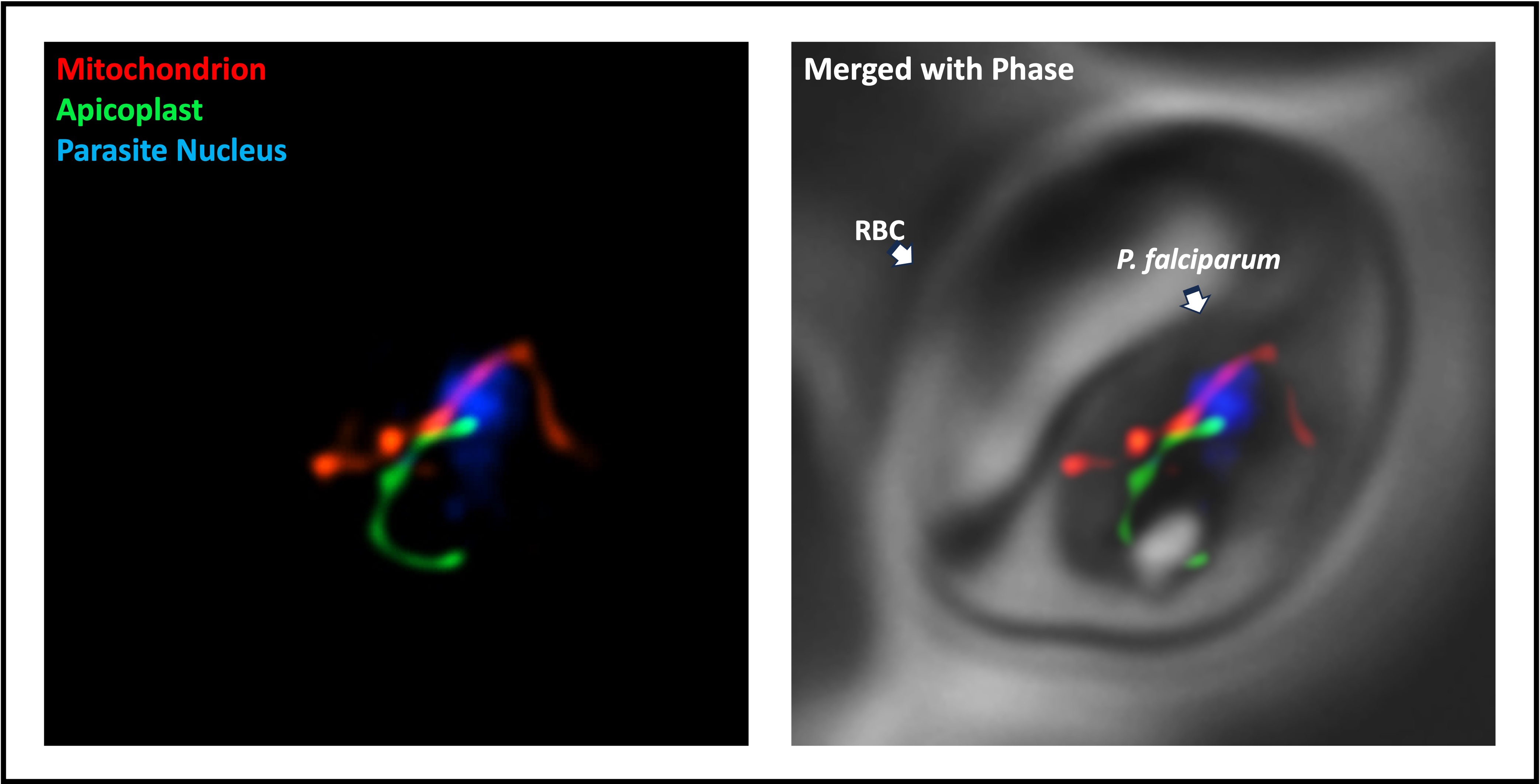
Organelle biology: The Plasmodium parasite retains a complex intracellular structure that includes two endosymbionts: a mitochondrion that is very different from its human counterpart, and an unusual plastid called the apicoplast. We know that both organelles are essential for the parasite’s survival, but we don’t fully understand how they are organized, what proteins they contain, or how exactly they support the parasite. Studying tiny compartments within a cell comes with its own set of challenges, so we build and employ appropriate genetic tools and reporters to characterize their biology and metabolic contributions.
Cellular transport: Multiple membranes (7 in the case of the apicoplast!) separate the extracellular milieu from the malaria parasite’s lumen. We’d like to know how metabolites, ions and proteins traverse all these membranes to arrive at the appropriate location, and in some cases, at the appropriate time during parasite development. We characterize novel transport proteins by determining their cellular location, substrate specificity, and role in parasite metabolism. We also study how signal sequences in parasite proteins dictate their destination.
Selected publications:
1. Swift S, Elahi R, Rajaram K, Liu HB, Prigge ST. The Plasmodium falciparum apicoplast cysteine desulfurase provides sulfur for both iron sulfur cluster assembly and tRNA modification. eLife. 2023; 11:12: e84491.
2. Rajaram K, Tewari SG, Wallqvist A, Prigge ST. Metabolic changes accompanying the loss of fumarate hydratase and malate-quinone oxidoreductase in the asexual blood stage of Plasmodium falciparum. J Biol Chem. 2022; 101897.
3. Okada M, Rajaram K, Swift RP, Mixon A, Maschek JA, Prigge ST, Sigala PA. Critical role for isoprenoids in apicoplast biogenesis by malaria parasites. eLife2022;11: e73208.
4. Swift RP, Rajaram K, Keutcha C, Liu HB, Kwan B, Dziedzic A, Jedlicka AE, Prigge ST. The NTP generating activity of pyruvate kinase II is critical for apicoplast maintenance in Plasmodium falciparum. eLife 2020; 9: e50807.
5. Rajaram K, Liu H, Prigge ST. A redesigned TetR-aptamer system for control of gene expression in Plasmodium falciparum. mSphere 2020; 5: e00457-20.
