Birgit Alber
Associate Professor of Microbiology
417A Biological Sciences Building
Areas of Expertise
- Biochemistry of central carbon metabolism
Education
- B.S. University of Marburg
- Ph.D. Virginia Tech
- Postdoc, University of Freiburg
Research Interests
Rhodobacter sphaeroides transposon mutant strain collection
Purple non-sulfur bacteria are some of the most metabolically versatile organisms known. Rhodobacter sphaeroides, Rhodospirillum rubrum, Rhodopseudomonas palustris, etc.can use light as a source of energy (anoxygenic phototrophy) or organic and inorganic compounds as electron donors and acceptors (respiratory and fermentative growth). In addition, these bacteria are also able to use a very large spectrum of carbon sources ranging from carbon dioxide (autotrophic growth) to fermentative products generated by other organisms in the same habitat (heterotrophic growth).
Biochemistry of intermediary carbon metabolism
The versatile growth mode of the anoxygenic phototroph Rhodobacter sphaeroides allows the identification of novel metabolic and regulatory pathways controlling ( i) the entry of a given carbon substrate into central carbon metabolism through peripheral pathways and ( ii) the subsequent conversion of initial intermediates into all so-called precursor metabolites (acetyl-CoA, oxaloacetate, pyruvate, phosphoenolpyruvate, alpha-ketoglutarate) from which the synthesis of cell constituents starts. The latter process is referred to as intermediary metabolism. R. sphaeroides is ideally suited for these studies because a given carbon substrate is soley used for synthesis of cell mass during anoxygenic photoheterotrophic growth. Our ultimate goal is to understand how precursor metabolites are provided in a defined ratio to ensure consistent cell composition during growth with different carbon substrates.
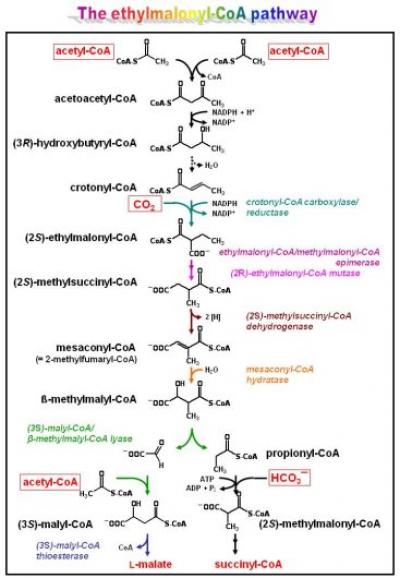
The ethylmalonyl-CoA pathway
One important route in intermediary metabolism of R. sphaeroides is the ethylmalonyl-CoA pathway (see Figure). It is required for growth withorganic substrates that are metabolized via the C2-compound acetyl-CoA (examples are fatty acids, alcohols, and esters, including various fermentation products, but also waxes, alkenes, taurine, and methylated compounds). Wheras many organisms (including Escherichia coli) use the glyoxylate cycle for acetyl-CoA assimilation, R. sphaeroides (and many other alpha-proteobacteria and actinomyces) uses the more recently elucidated ethylmalonyl-CoA pathway. The key enzyme of the ethylmalonyl-CoA pathway is a novel carboxylase catalyzing the reductive carboxylation of an enoyl-CoA substrate: crotonyl-CoA carboxylase/reductase. The enzyme, for which the catalytic mechanism is under investigation, connects the C 4- and C 5-branch of the pathway. Other reactions of the ethylmalonyl-CoA pathway are also unique. We use biochemical and genetic approaches to study the individual enzymatic reactions involved as well as how flow through the pathway is regulated. The latter is of particular interest, as the ethylmalonyl-CoA pathway co-assimilates transiently generated carbon dioxide and, therefore, allows for efficient carbon usage.
3-Hydroxypropionate metabolism
The C3-compound 3-hydroxypropionate has only recently been recognized as an important intermediate in the carbon cycle. For R. sphaeroides, 3-hydroxypropionate is reductively converted via acrylyl-CoA to propionyl-CoA involving an acrylyl-CoA reductase. Oxidation of 3-hydroxypropionate to acetyl-CoA and carbon dioxide may also occur. As a consequence carbon has to be partionioned correctly between the oxidative and reductive route in order to lead to a defined ratio of precursor metabolites that ensures consistent cell composition.
Relevant Publications
Schneider K, Asao M, Carter MS, & Alber BE. (2012). Rhodobacter sphaeroides uses a reductive route via propionyl-CoA to assimilate 3-hydroxypropionate. J. Bacteriol. 194,225-232.
Laguna R, Tabita FR, & Alber BE. (2011) Acetate-dependent photoheterotrophic growth and the differential requirement for the Calvin-Benson-Bassham reductive pentose phosphate cycle in Rhodobacter sphaeroides and Rhodopseudomonas palustris. Arch Microbiol. 193, 151-154.
Alber BE. ( 2011) Biotechnological potential of the ethylmalonyl-CoA pathway. Appl Microbiol Biotechnol. 89,17-25 .
Erb TJ, Frerichs-Revermann L, Fuchs G, & Alber BE. (2010) The apparent malate synthase activity of Rhodobacter sphaeroides due to two paralogous enzymes, (3 S)-malyl-coenzyme A (CoA)/beta-methylmalyl-CoA lyase and (3 S)-malyl-CoA thioesterase. J Bacteriol. 192 , 1249-58 .
Erb TJ, Fuchs G, & Alber BE. (2009) (2S)-Methylsuccinyl-CoA dehydrogenase closes the ethylmalonyl-CoA pathway for acetyl-CoA assimilation. >Mol Microbiol. 73 , 992-1008 (Comment in the same issue).
Erb TJ, Brecht V, Fuchs G, Müller M, & Alber BE. (2009) Carboxylation mechanism and stereochemistry of crotonyl-CoA carboxylase/reductase, a carboxylating enoyl-thioester reductase. Proc Natl Acad Sci U S A. 106, 8871-6 .
Erb TJ, Rétey J, Fuchs G, & Alber BE. (2008) Ethylmalonyl-CoA mutase from Rhodobacter sphaeroides defines a new subclass of coenzyme B 12-dependent acyl-CoA mutases. J Biol Chem . 283,32283-93 .
Alber BE , Kung JW, & Fuchs G. (2008) 3-Hydroxypropionyl-coenzyme A synthase from Metallosphaera sedula, an enzyme involved in autotrophic CO 2 fixation. J. Bacteriol. 190 ,1383-9 .
Zarzycki J, Schlichting A, Strychalsky N, Müller M, Alber BE , & Fuchs G. (2008) Mesaconyl-coenzyme A hydratase, a new enzyme of two central carbon metabolic pathways in bacteria . >J Bacteriol. 190 ,1366-74 .
The all-time favorites:
Erb TJ, Berg IA, Brecht V, Müller M, Fuchs G, & Alber BE. (2007) Synthesis of C 5-dicarboxylic acids from C 2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway . Proc Natl Acad Sci U S A. 104,10631-6 .
Alber BE & Fuchs G. (2002) Propionyl-coenzyme A synthase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO 2 fixation. J Biol Chem. 277,12137-43 .
Alber BE& Ferry JG. (1994) A carbonic anhydrase from the archaeon Methanosarcina thermophila. Proc Natl Acad Sci., U S A., 6909-13.
